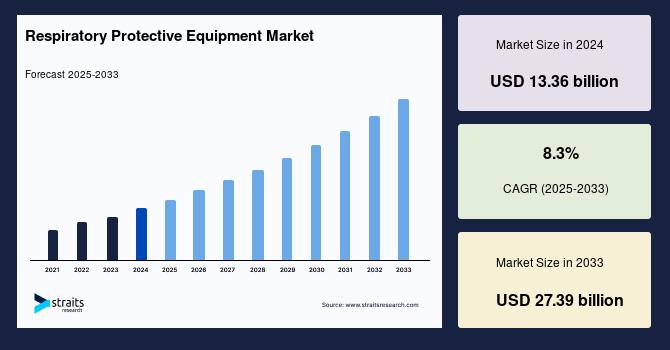
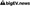The increasing complexity of biopharmaceutical manufacturing processes has heightened the importance of viral clearance and safety measures. As the industry faces evolving viral threats, regulatory expectations are also intensifying, necessitating robust strategies to ensure product safety. The challenge lies in balancing the need for rapid production with stringent safety protocols, as any lapse could lead to significant public health risks and regulatory repercussions. Companies must navigate a landscape marked by emerging viral pathogens and the potential for contamination, making viral clearance a critical focus area for biopharmaceutical developers.
To address these challenges, the industry is adopting advanced viral clearance technologies and methodologies, emphasizing the need for comprehensive risk assessments and validation studies. Key insights reveal that integrating innovative approaches, such as high-throughput screening and real-time monitoring, can enhance viral safety protocols. Furthermore, collaboration among stakeholders, including regulatory bodies and industry leaders, is essential to establish best practices and harmonize standards. Ultimately, prioritizing viral safety not only protects public health but also fosters trust in biopharmaceutical products, reinforcing the industry's commitment to quality and safety in a rapidly evolving landscape.