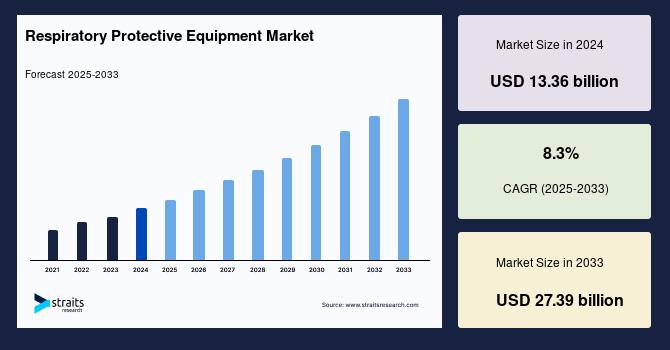
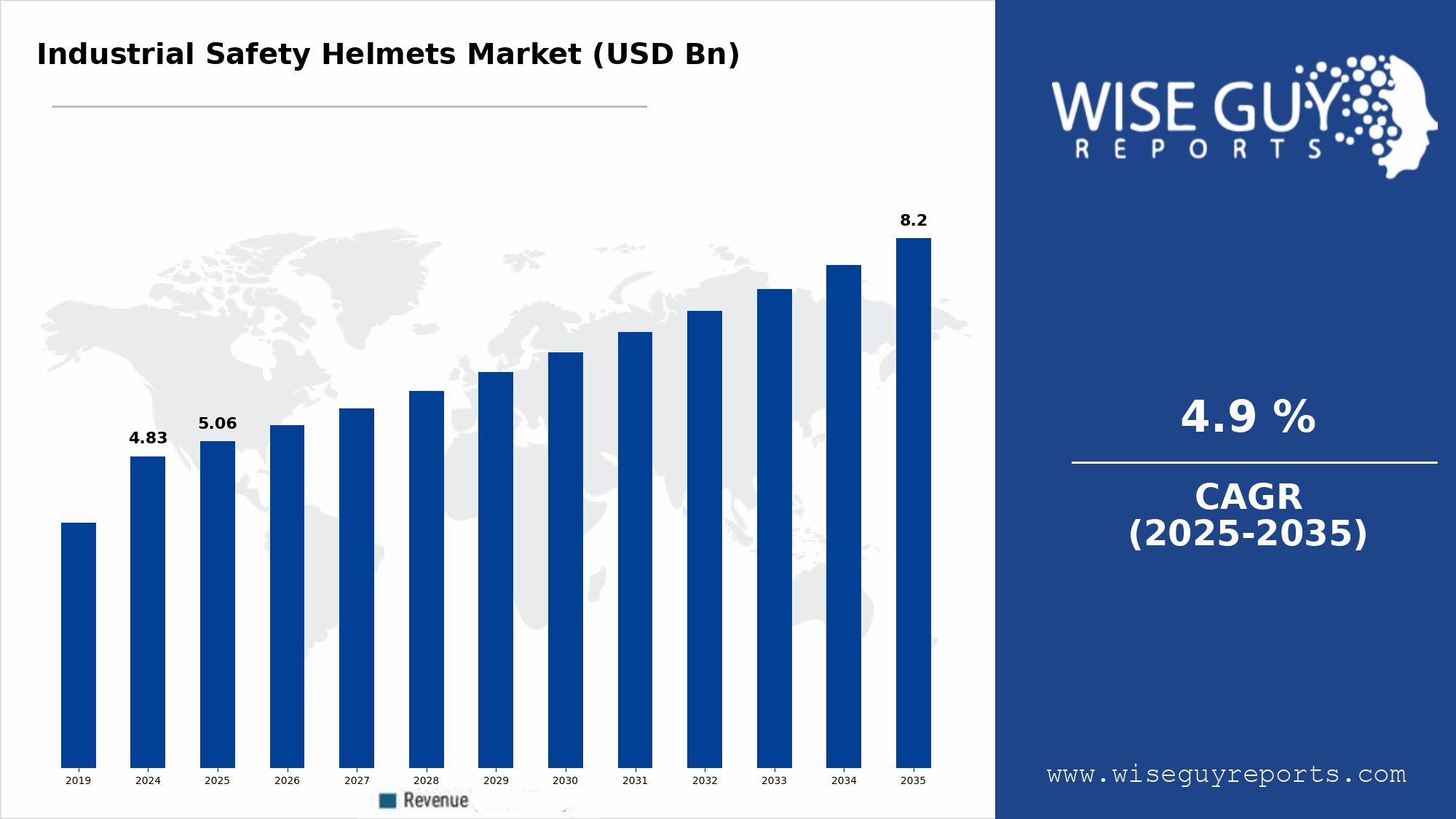
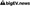Data integrity and quality are paramount for drug discovery, manufacturing efficiency, regulatory compliance, and patient safety. As the pharmaceutical industry increasingly integrates artificial intelligence (AI) into its processes, the need for robust data governance becomes critical. The challenge lies in ensuring that the vast amounts of data generated are not only accurate but also secure and compliant with regulatory standards. This intersection of AI and data governance presents a dual-edged sword: while AI can enhance operational efficiencies and decision-making, it also raises concerns about data management practices and potential biases in algorithms that could impact drug development outcomes.
To navigate these complexities, organizations must adopt comprehensive data governance frameworks that prioritize transparency, accountability, and ethical considerations in AI applications. Key insights suggest that establishing clear protocols for data management, coupled with continuous monitoring and validation processes, can mitigate risks associated with AI-driven initiatives. The implications are profound: by fostering a culture of data integrity and embracing innovative governance strategies, the pharmaceutical sector can enhance its operational resilience, ultimately leading to improved patient outcomes and regulatory compliance.