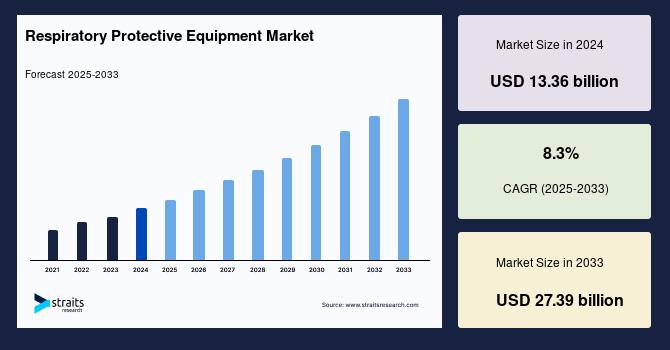
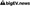Data integrity and quality are paramount for drug discovery, manufacturing efficiency, regulatory compliance, and patient safety. As the pharmaceutical industry increasingly integrates artificial intelligence (AI) into its processes, the challenge of maintaining robust data governance becomes critical. The intersection of AI and data management raises concerns about the reliability of data used in decision-making, which can directly impact drug efficacy and safety. Ensuring that data remains accurate and secure is not just a regulatory requirement; it is essential for fostering trust among stakeholders, including patients, healthcare providers, and regulatory bodies. The evolving landscape necessitates a reevaluation of existing data governance frameworks to accommodate the complexities introduced by AI technologies.
To address these challenges, organizations must adopt a proactive approach to data governance that emphasizes transparency, accountability, and continuous improvement. Implementing comprehensive data management strategies will enable companies to harness the full potential of AI while mitigating risks associated with data misuse or inaccuracies. Key insights suggest that fostering a culture of data stewardship, investing in advanced analytics, and prioritizing regulatory compliance will be crucial for navigating the future of drug manufacturing. By aligning AI capabilities with stringent data governance practices, the industry can enhance operational efficiencies and ultimately improve patient outcomes.